First Class Design for Protection of the Environment
SULPHUR TO SULPHURIC ACID
Rameshni & Associates Technology & Engineering (RATE) offers Licensing technologies in sulphur technologies and tail gas treating and converting of sulphur to sulphuric acid for upstream, to midstream and downstream with full performance guarantees.
The large volumes of sulphur are produced around the world that requires shipment where to be used in fertilizer and agriculture business. Sulphur can be burned to produce the sulphuric acid.
The major use of Sulphuric acid is in the production of fertilizers, e.g., superphosphate of lime and ammonium sulfate. It is also widely used in the manufacture of chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, and drugs. Petroleum refining is another destination of Sulphuric acid, where it is used to wash impurities out of gasoline and other refinery products.

Sulphuric acid is used as a dissolving medium in metal extraction processes (such as Copper, Nickel). Within the same process, during what is known as ‘Roasting’, the part of the Sulphur deposits present in the medium are removed as Sulphur Dioxide, along with other gases, which are collectively referred to as ‘smelting gas’. Since Sulphur Dioxide is also a state through which Sulphuric manufacturing processes proceed, this is considered also as a source of ‘raw material’ as far as Sulphuric production is considered.
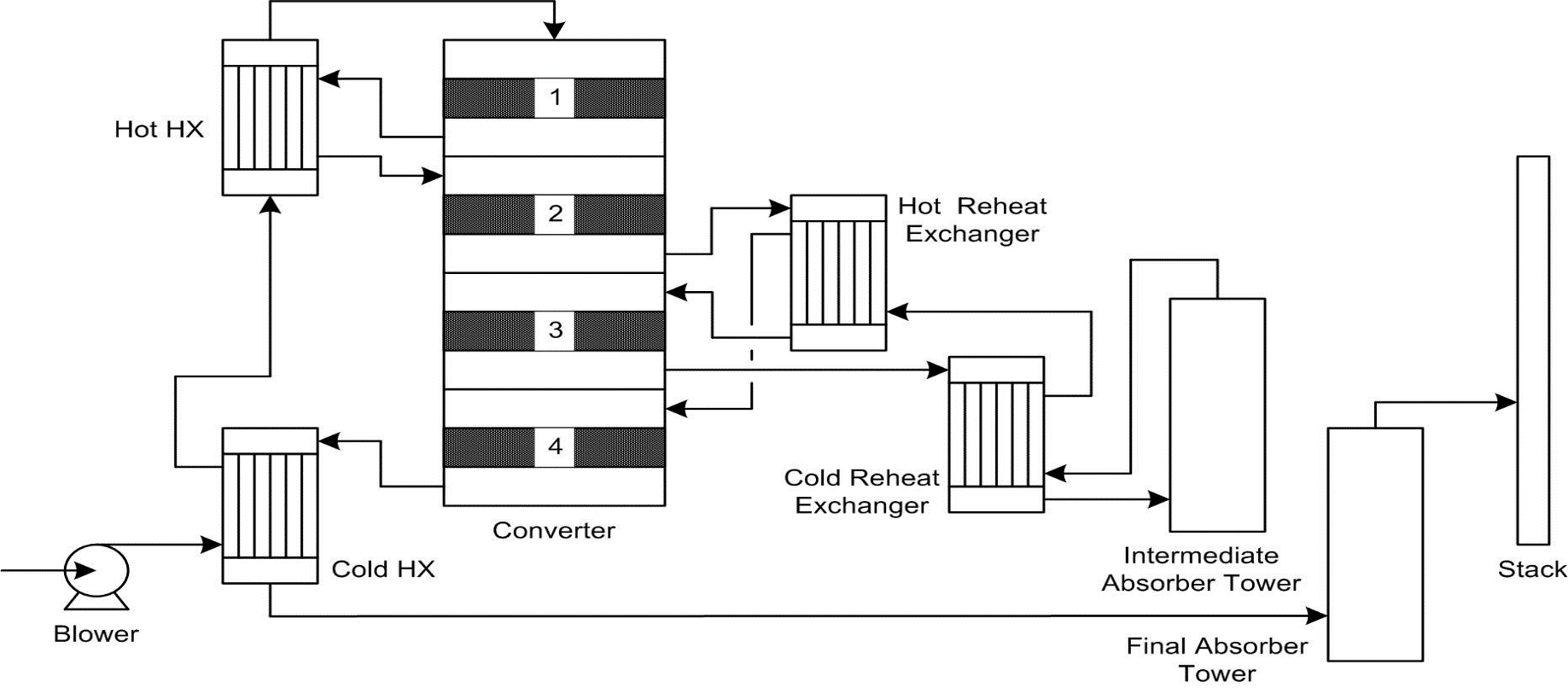
Re-melter and sulphur burner
- The solid sulphur is re-melted by using heating steam coil. The melting of Sulphur is performed separately without contact of the air for oxidation into SO2. The molten Sulphur is supplied to a burner along with a countercurrent flow of dry air to enable the oxidation reaction and produce Sulphur Dioxide.
Air Dryer
- Air from the air blower shall be dried by the air dryer before use.
Waste heat boiler
- to recover the energy and cool the burner outlet
- 4 Pass Converter- Catalyst would increase rate of conversion and increased SO3 formation. V2O5 (vanadium pentoxide) – can last up to 20 years

Absorbing Tower
- Absorption of SO3 into H2SO4 and Recirculated H2SO4 is cooled
- Also known as Double Contact/Double Absorption
- Characterized by two SO3 absorption towers
- Double absorption plants are able to meet current emission restrictions for SO2